6+ Chapter 13 Review Ions In Aqueous Solutions And Colligative Properties
Click here to access free chemistry study material. The relative strength of an acid or base is the extent to which it ionizes when dissolved in water.

Chemical Principles The Quest For Insight Atkins P Jones L
Solutions of hydrogen in palladium may be formed by exposing Pd metal to H 2 gas.
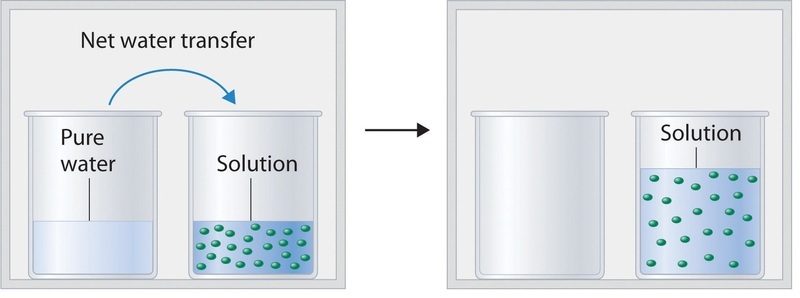
. Reverse osmosis is the process in which pressure is applied to overcome colligative property and osmotic pressure that is. Hit the previous tab until you get to the beginning of the textbook problems. The process NaC 2 H 3 O 2 a q NaC 2 H 3 O 2 s NaC 2 H 3 O 2 a q NaC 2 H 3 O 2 s is exothermic and the heat produced by this.
It is used to remove ions minerals chemicals and impurities from water. Solutions and Colligative properties khali29. In this solution an excess of solid AgCl dissolves and dissociates to produce aqueous Ag and Cl ions at the same rate that these aqueous ions combine and precipitate to form solid AgCl Figure 152.
Watch the video and take notes. Lesson 6 - Using Colligative Properties to Determine Molar Mass Using Colligative Properties to Determine Molar Mass. Apchemunit11presentation 120104190723-phpapp01 Cleophas Rwema.
NCERT Solutions for Class 10 Maths Chapter 14 More. NCERT Solutions for Class 10 Maths Chapter 13. NCERT Solutions for Class 10 Maths Chapter 13.
BCS High solubility The highest single unit dose is completely soluble in 250 ml or less of aqueous solution at pH 1 - 68 37. Common abbreviations include s for solids l for liquids g for gases and aq for substances dissolved in water aqueous solutions as introduced in the preceding chapter. Under certain conditions 094 g of hydrogen gas is dissolved in 215 g of palladium metal solution density 108 g cm 3.
TITRIMETRIC MTHODS Titrimetric methods are widely used in chemistry to determine oxidants reductants acids bases metal ions etc. Because silver chloride is a sparingly soluble salt the equilibrium concentration of its dissolved ions in the solution is relatively low. The development of pharmaceutical technology in past years has presented the development of alternative dosage forms for patients who may have difficulty in swallowing of conventional tablets.
NCERT Solutions for Class 10 Science. Fast Disintegrating Drug Delivery Systems. However although the last digit 5 is not.
300 cm 3 of an aqueous solution contains 156g of a polymer. NCERT Solutions for Class 10 Science. Shift In Equilibrium Between Ferric Ions And Thiocyanate Ions Experiment.
There is far more hydrogen carbonate ion HCO 3 in blood than in seawater. Kinetics 6 hours lectures 3 hours seminars. Learn about Chemistry its branches and the key concepts covered under the subject at the K-12 level.
The solubility guidelines in Table 41 may be used to predict whether a precipitation reaction will occur when solutions of soluble ionic compounds are mixed together. C Titrant volume 2500 mL. Chemistry is the study of matter its properties how and why substances combine or separate to form other substances and how substances interact with energy.
Go to chapter Atoms Elements Ions. You can use page 13 to review. Go to Holt McDougal Modern Chemistry Chapter 13.
Reaction kinetics in relation to reaction mechanism. Relative lowering of vapour pressure boiling point elevation and boiling point depression in binary solutions containing non-volatile solutes. If the ionization reaction is essentially complete the acid or base is termed strong.
Sigma And Pi Bond. 2013 Rajendra Awasthi. NCERT Solutions for Class 10 Maths Chapter 14.
AP Chemistry Chapter 13 Outline. If relatively little ionization occurs the acid or base is weakAs will be evident throughout the remainder of this chapter there are many more weak acids and bases. Calculate the number of moles and the mass of the solute in each of the following solutions.
Chemical Reactions in Aqueous. This titrant addition involves a stoichiometric amount of base the equivalence point and so only products of the neutralization reaction are in solution water and NaClNeither the cation nor the anion of this salt undergo acid-base ionization. Bending the disk creates nucleation sites around which the metastable NaC 2 H 3 O 2 quickly crystallizes a later chapter on solutions will investigate saturation and supersaturation in more detail.
A 200 L of 185 M H 2 SO 4 concentrated sulfuric acid b 1000 mL of 38 10 6 M NaCN the minimum lethal concentration of sodium cyanide in blood serum c 550 L of 133 M H 2 CO the formaldehyde used to fix tissue samples. NCERT Solutions for Class 10 Science. Go to this page.
The concentration of hydrogen in the palladium depends on the pressure of H 2 gas applied but in a more complex fashion than can be described by Henrys law. Titration is based on a reaction between the analyte unknown sample and the regent of known concentration and reaction stoichiometry. Find step-by-step solutions and answers to Modern Chemistry - 9780547586632 as well as thousands of textbooks so you can move forward with confidence.
Print the note taking guide on molarity and colligative properties. Colligative Properties Relative Lowering Of Vapour Pressure. NCERT Solutions for Class 10 Maths Chapter 13.
Question Stem for Questions 9 and 10. Click on Chapter 6. To this solution A an equal volume of 01 molal aqueous barium chloride solution is added to make a new solution B.
The theoretical values of molecular mass when calculated from the colligative properties of solutions are sometimes found to differ from the experimentally obtained valuesThese values are often referred to as abnormal molar masses. Go to Holt McDougal Modern Chemistry Chapter 6. In the previous example if table salt NaCl were added to the solution before or after adding eqPbCl_2 eq less would dissolve into the water because there would be chloride ions more.
Most ions are more abundant in seawater than they are in blood with some notable exceptions. The boiling point of water in a 01 molal silver nitrate solution solution A is x ºC. The only process generating hydronium ions is the autoprotolysis of water.
A Review with Special Emphasis on Fast Disintegrating Tablets. The physical states of reactants and products in chemical equations very often are indicated with a parenthetical abbreviation following the formulas. When performing calculations stepwise as in Example 317 it is important to refrain from rounding any intermediate calculation results which can lead to rounding errors in the final resultIn Example 317 the molar amount of NaCl computed in the first step 1325 mol would be properly rounded to 132 mol if it were to be reported.
One merely needs to identify all the ions present in the solution and then consider if possible cationanion pairing could result in an insoluble compound. NCERT Solutions for Class 10 Maths Chapter 14. Solutions colligative properties are properties that depend on the concentration of molecules or ions of the solute but not on the.
Acid and Base Ionization Constants. Solubility and distribution phenomena. Indeed it is the third most common ion in bloodThis difference is significant because the HCO 3 ion and some related species CO 3 2 CO 2 aq have an important role in.
These notations are. Vant Hoff explained that when solutes are dissolved in a solvent they dissociate into ions. Check your answers to the video quiz near the bottom of page 10.
Ions in Aqueous Solutions and Colligative Properties.

Chapter Ions In Aqueous Solutions And Colligative

Introduction To Chemistry General Organic And Biological Pdf Chemical Bond Chemical Reactions

How Do Aqueous Solutions Of Ionic Molecular Compounds Differ Study Com

Chapter 13 Ions In Aqueous Solutions And Colligative Properties Video Solutions Holt Modern Chemistry Numerade

Which Of The Following Aqueous Solutions Has The Highest Boiling Point

Chemical Principles The Quest For Insight Atkins P Jones L

Pdf Modeling Chemical Reactivity In Ionic Detergent Micelles A Review Of Fundamentals
Chapter 13 Ions In Aqueous Solutions And Colligative Properties Flashcards Quizlet

Colligative Properties Definition Types Examples Raoult S Law

Sp18 Sample Quiz 2 Colligative Properties Key General Chemistry 010 Spring 2018 Sample Quiz Studocu

Chapter 13 Ions In Aqueous Solutions And Colligative Properties Video Solutions Holt Modern Chemistry Numerade

Ppt Honors Chemistry Ch 14 Ions In Aqueous Solutions And Colligative Properties Powerpoint Presentation Id 2431285

Chem Ch 13 Ions In Aqueous Solutions And Colligative Properties Flashcards Quizlet

Pdf Cliffsstudysolver Chemistry Mostafa Daif Academia Edu

Chapter 13 Preview Multiple Choice Short Answer Ppt Video Online Download

Chapter 13 Ions In Aqueous Solutions And Colligative Properties Ppt Download
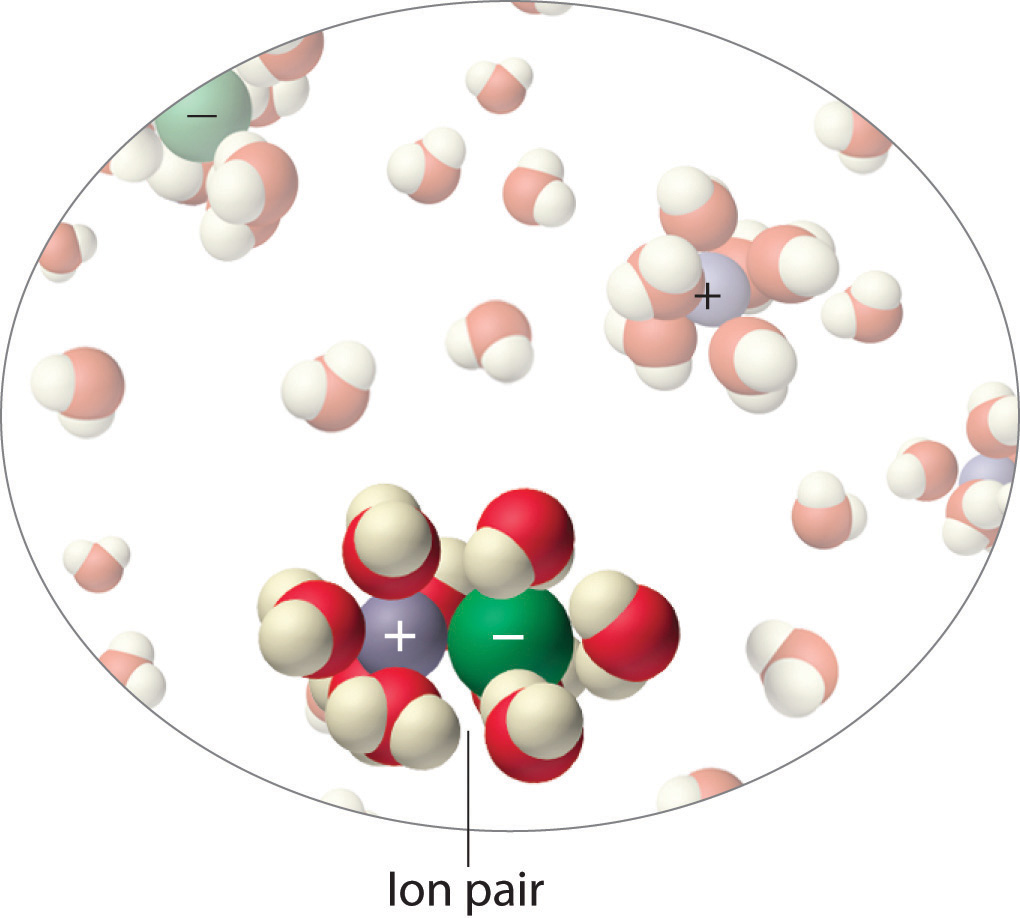
Colligative Properties Of Solutions